.PNG)
Gas Laws Presentation Chemistry
Graphical Representation of Boyle's Law. Graph of pressure vs volume; Graph of pressure vs 1/volume; Application of Boyle's Law. Syringe operation - A syringe operates according to the principles of Boyle's system. When we pull on the syringe's plunger, we increase the barrel's capacity, which lowers the pressure inside of it and.

The graph for Boyle's law is called
We can use Boyle's law to predict what will happen to the volume of a sample of gas as we change the pressure. Because PV is a constant for any given sample of gas (at constant T), we can imagine two states; an initial state with a certain pressure and volume (P 1 V 1), and a final state with different values for pressure and volume (P 2 V 2).Because PV is always a constant, we can equate.
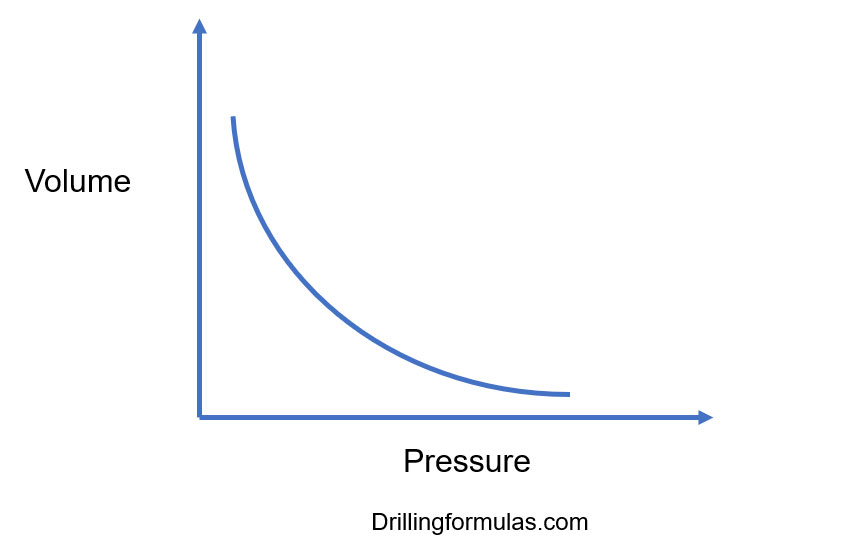
Boyle’s Gas Law and Its Application in Drilling
Suppose the gas with pressure P 1 and volume V 1 expands or shrinks to pressure P 2 and volume V 2. Then, using Boyle's law equation, P 1 V 1 = k and P 2 V 2 = k. From the above two equations. P 1 V 1 = P 2 V 2. This equation shows that as the pressure increases, the volume decreases and vice versa. For example, when the pressure doubles, the.

Boyle's Law — Overview & Formula Expii
Boyle's law, a relation concerning the compression and expansion of a gas at constant temperature. This empirical relation, formulated by the physicist Robert Boyle in 1662, states that the pressure ( p) of a given quantity of gas varies inversely with its volume ( v) at constant temperature; i.e., in equation form, pv = k, a constant.

AP Chemistry Boyle’s Law Soxteacher
Charles's law implies that the volume of a gas is directly proportional to its absolute temperature. 5.3: The Simple Gas Laws- Boyle's Law, Charles's Law and Avogadro's Law is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The volume of a gas is inversely proportional to its pressure and.
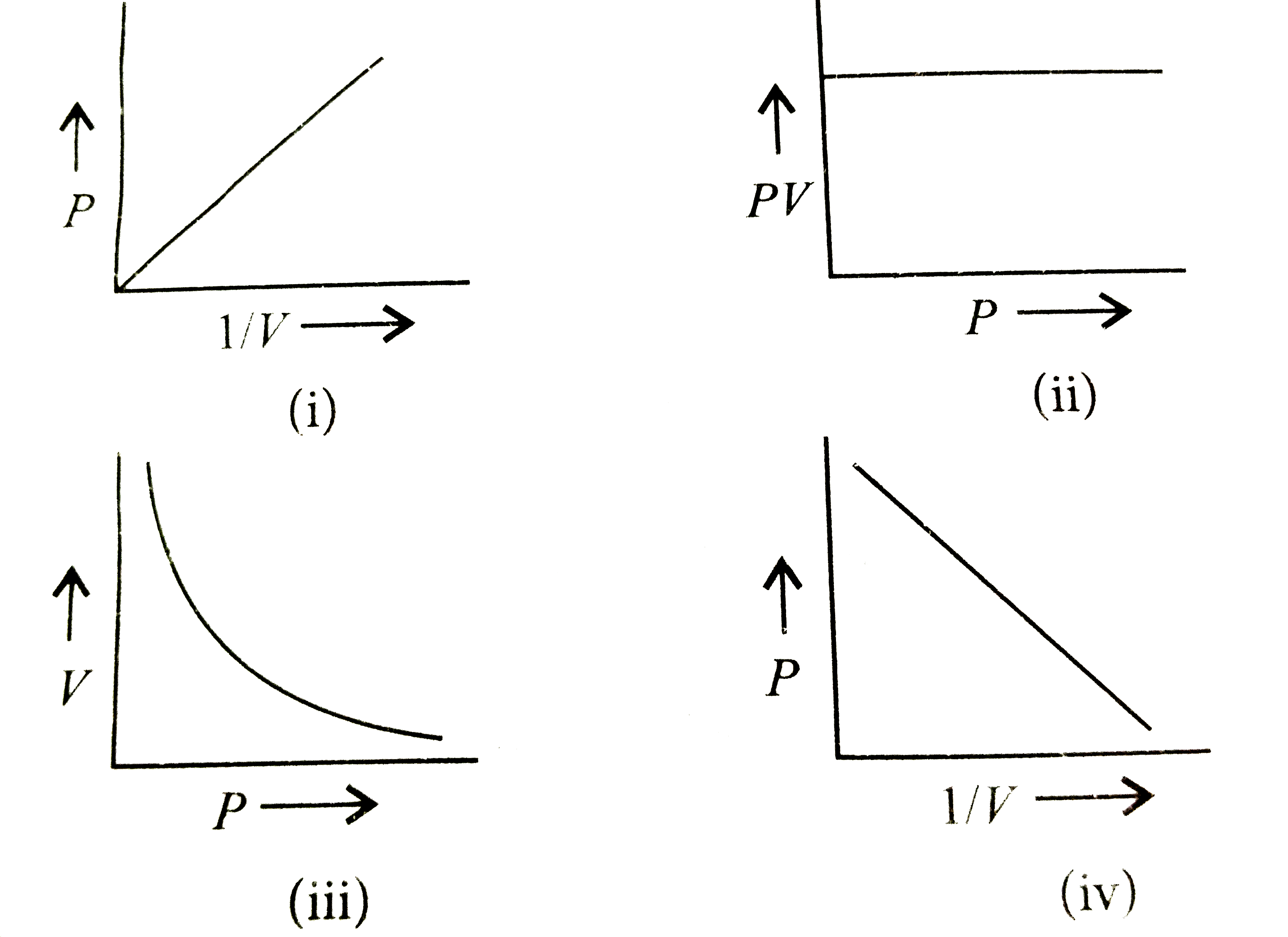
Which of the following graphs represents Boyle's law correctly? (i)
This expression can be obtained from the pressure-volume relationship suggested by Boyle's law. For a fixed amount of gas kept at a constant temperature, PV = k. Therefore, P1V1 = k (initial pressure * initial volume) P2V2 = k (final pressure * final volume) ∴ P1V1 = P2V2. This equation can be used to predict the increase in the pressure.
Suka Chemistry Boyle's law definition
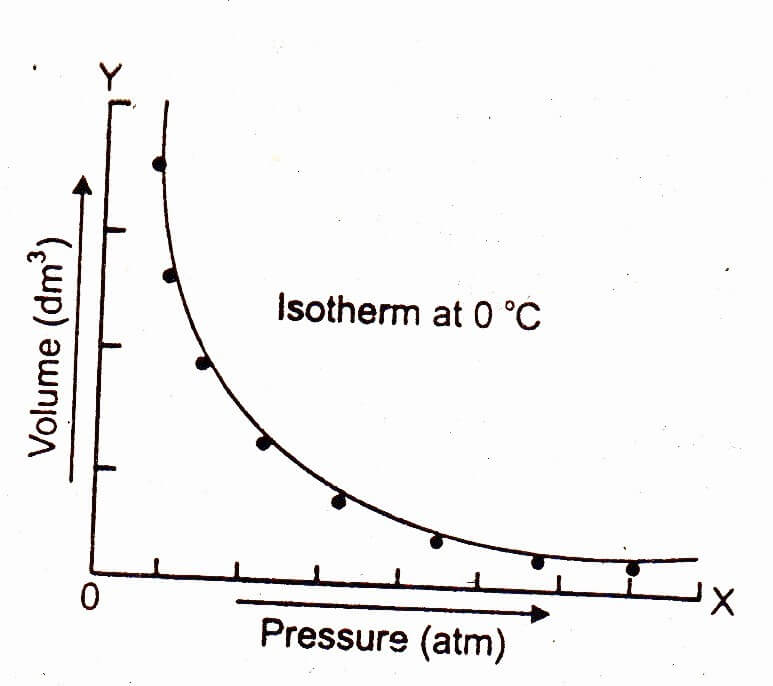
Figure 14.3.1 14.3. 1: Robert Boyle. (CC BY-NC; CK-12) Mathematically, Boyle's law can be expressed by the equation: P × V = k P × V = k. The k k is a constant for a given sample of gas and depends only on the mass of the gas and the temperature. The table below shows pressure and volume data for a set amount of gas at a constant temperature.

Mr Toogood Physics The Experimental gas laws
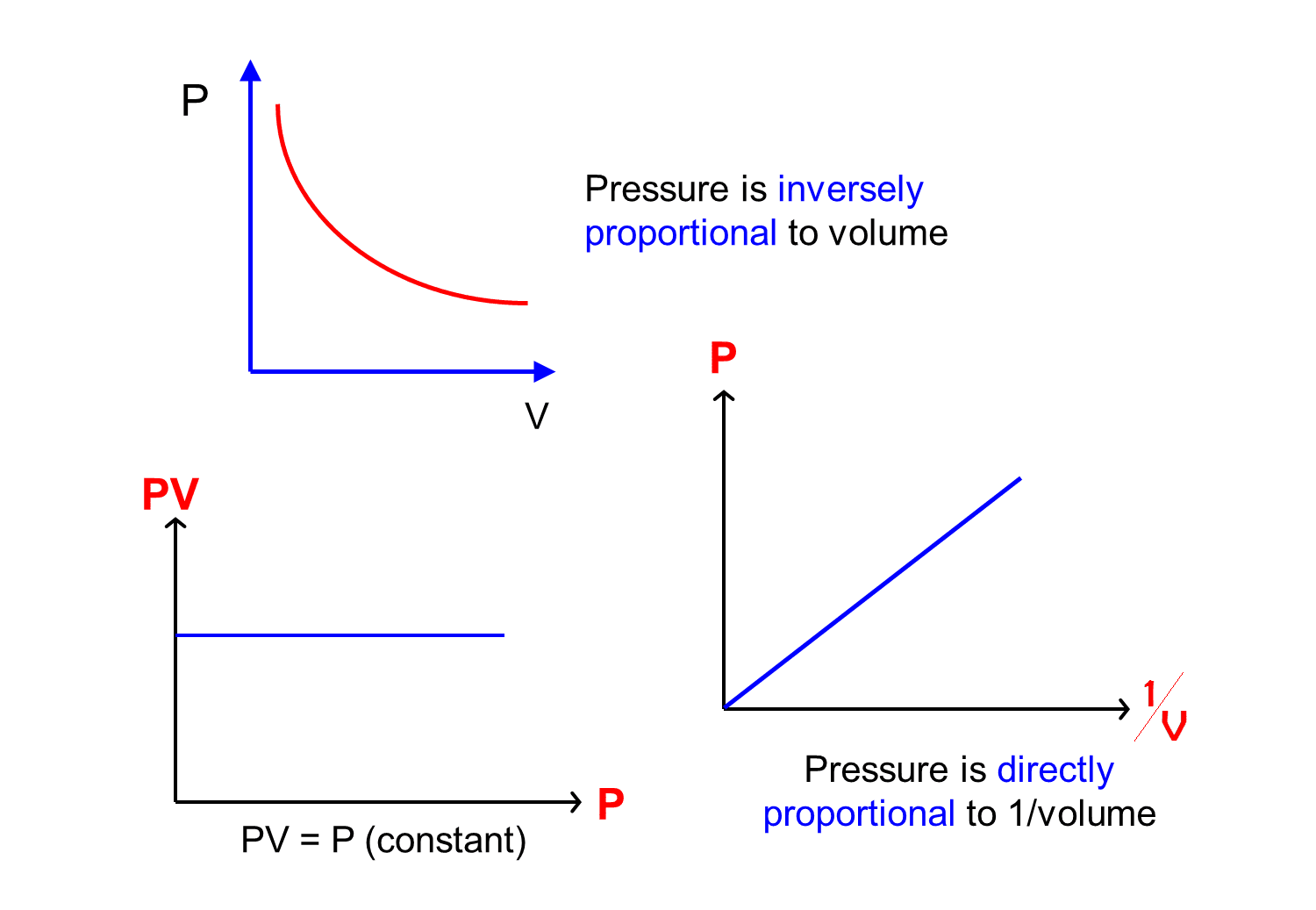
Boyle's Law Graph. There are two conventional graphs to represent Boyle's law. Pressure v/s volume. Pressure v/s volume⁻¹; Graph 1 - Pressure v/s Volume: Represents graph of question pV=K. The value of K is different for each mass of gas, it varies only with respect to temperature.
Boyle' Law
Boyle's Law defines the pressure-volume relationship of a gas at constant temperature and mass. Boyle's formula is P 1 V 1 = P 2 V 2 (thus, Initial pressure * Initial volume = Final pressure * Final volume). Pressure- inverse Volume graph is a straight line. But, the pressure-volume graph gives a curve.

8 Boyle’s Law Examples in Real Life StudiousGuy
This Boyle's law calculator works in any direction you like. Just insert any three parameters, and the fourth one will be calculated immediately! We can visualize the whole process on Boyle's law graph. The most commonly used type is where the pressure is a volume function. For this process, the curve is a hyperbola.

What are boyles law? Definition, Types and Importance physics AESL
The graph of Boyle's law is known as a pressure-volume graph or PV curve. The pressure and volume graph at constant temperature is known as an isotherm. As the Pressure increases with decreasing in volume, and vice versa. Other parameters such as temperature and amount of gas are constant in the graph. The equation of the curve is PV = K.

What are boyles law? Definition, Types and Importance physics AESL
Robert Boyle's observations are summed up in Boyle's law, which states that for a given mass of gas at constant temperature, the volume of a gas varies inversely with pressure. Because of the inverse relationship, the product of the two quantities, pressure and volume, is constant. When given any two sets of pressure and volume, at a given.
:max_bytes(150000):strip_icc()/boylesdatagraphed-56a129b33df78cf77267fe5d.jpg)
Boyle's Law Worked Sample Chemistry Problem
Explore math with our beautiful, free online graphing calculator. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more.

PPT Graphing Boyle’s Law PowerPoint Presentation, free download ID
Bookshelf ID: NBK538183 PMID: 30844210. Boyle's law is a gas law that describes the relationship between the pressure and volume of gas for a mass and temperature. This law is the mechanism by which the human respiratory system functions. Boyle's law is equivalent to PV = K (P is pressure, V is volume, K is a constant), or one may state.
Boyle’s Law Chemistry Skills
Explore Robert Boyle's 17th-century experiments with gases that led to Boyle's Law. Discover how Boyle's Law, demonstrating the inverse relationship between gas pressure and volume, laid the groundwork for the ideal gas equation. This historical journey illuminates key principles in gas behavior. Created by Ryan Scott Patton.
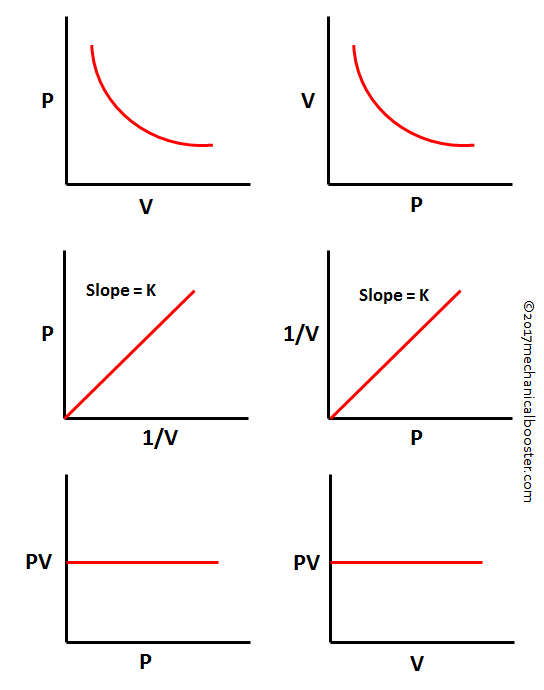
Boyle's Law Statement, Formula Example and Graph Mechanical Booster
Boyle's Law Example Problem. For example, calculate the final volume of a balloon if it has a volume of 2.0 L and pressure of 2 atmospheres and the pressure is reduced to 1 atmosphere. Assume temperature remains constant. P 1 V 1 = P 2 V 2. (2 atm) (2.0 L) = (1 atm)V 2.